What You Should Know: - Genomics plc, a leader in polygenic risk score (PRS) technology, and pharmaceutical giant GSK inks a new collaboration to explore the potential of PRS for optimizing clinical trial design. - The innovative approach could revolutionize the way researchers select patients and ultimately lead to faster development of new treatments. Unlocking the Power of Genetics for Clinical Trials Genomics plc brings its expertise in PRS technology to the table.
Read More
Clinical Trials | News, Analysis, Insights - HIT Consultant
myTomorrows Partners with brainstrust to Support Brain Cancer Patients in Accessing Clinical Trials
What You Should Know: myTomorrows, a global health technology company connecting patients with all possible treatment options, today announced a new partnership with brainstrust, a UK-based charity that helps people living with a brain tumor reach their potential and thrive.Under the new partnership, people with brain tumors will be equipped with the means to bolster their knowledge of relevant treatment options, potentially extending to participation in clinical trials and access to
Read More
Pi Health Secures $30M to Democratize Cancer Clinical Trials
What You Should Know: - Pi Health, a newly independent oncology-focused clinical research company raises $30M in Series A funding led by AlleyCorp and Obvious Ventures, with additional investments from Invus Capital and leaders in oncology from across the globe. - The company, previously incubated by BeiGene (NASDAQ: BGNE), aims to revolutionize access to innovative cancer treatments through its technology platform and global network of clinical trial sites. Less Than 8% of
Read More
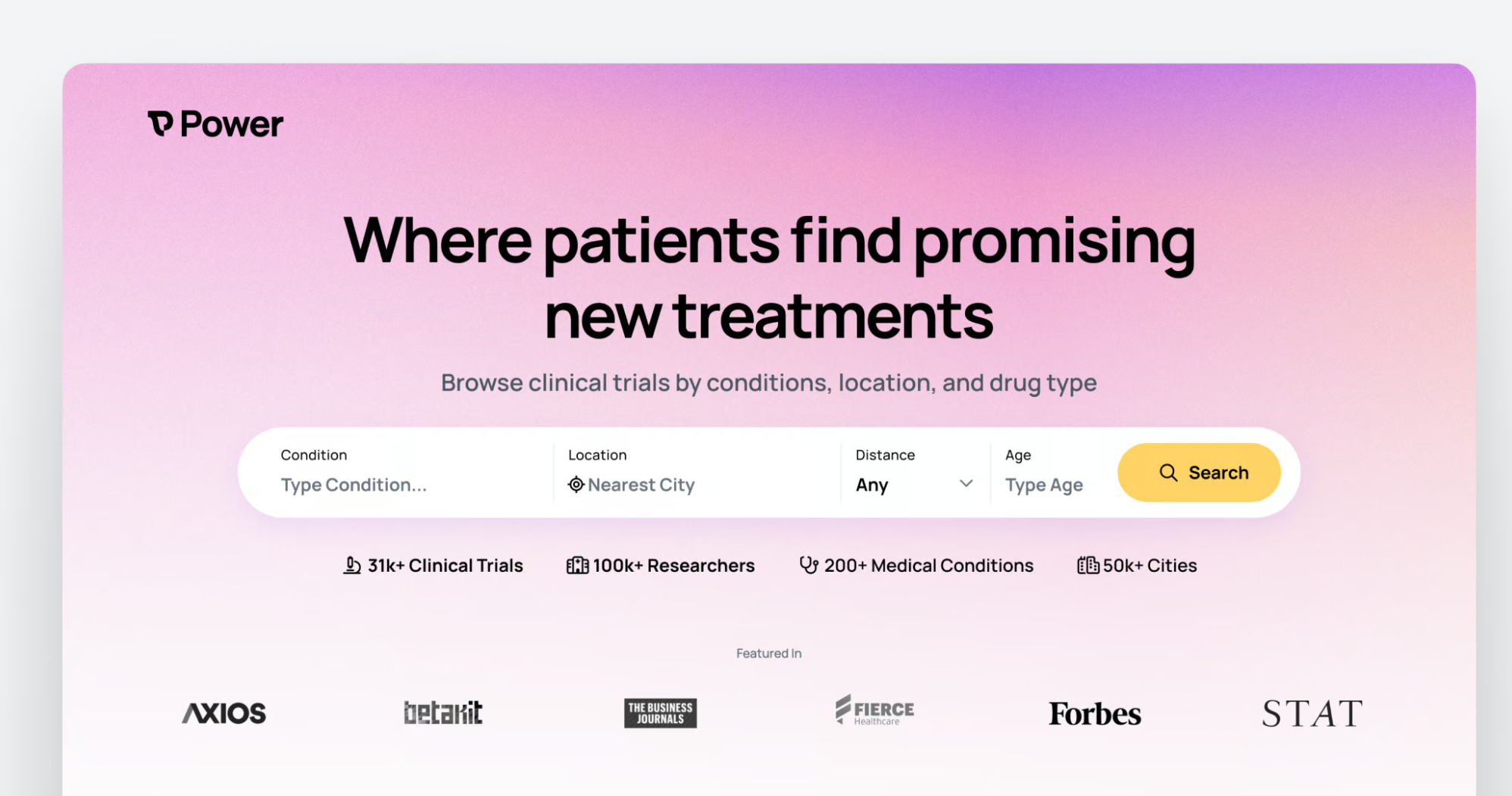
Power Secures $11.9M to Democratize Clinical Trials
What You Should Know: - Power, the company transforming the traditional clinical trial landscape, announced today the close of their $11.9M Series A funding round led by Kin, Contrary, and Footwork. - The strategic funding fuels Power's mission to democratize access to clinical trials and diversify the pool of participants, ultimately accelerating life-saving medical research. Revolutionizing Clinical Trial Participation Founded in 2021 by Brandon Li and Michael Gill, Power
Read More
How Open-Source Technologies Will Transform Digital Clinical Trials
Although the traditional nearly decade-long clinical trial timelines completely collapsed during COVID, this truncating was not necessarily novel. Precedent exists for accelerating approval timelines during health emergencies such as the AIDs/HIV epidemic, where Compound S, a re-make of AZT, or azidothymidine, originally developed to fight cancer, became controversially fast-tracked after the discovery that it could block HIV activity. Likewise, when potentially lifesaving drugs are
Read More
Unlearn Raises $50M to Accelerate AI-Powered Clinical Trials with Digital Twins
What You Should Know: - Unlearn®, a leading AI company using digital twins to revolutionize clinical trials, announced today a $50M Series C funding round led by Altimeter Capital. This investment, bringing Unlearn's total funding to over $130M, will fuel its mission to eliminate trial and error in medicine through faster, smaller, and more ethical clinical trials. - Unlearn collaborates with pharmaceutical companies, biotechs, and academic institutions to optimize and streamline clinical
Read More
H1 Unveils GenosAI Pro: Supercharging Clinical Trials with Conversational AI
What You Should Know: - H1, the go-to resource for healthcare professionals, clinical trial information, and life sciences research, announced the launch of its conversational AI tool GenosAI Pro. - The conversational AI tool empowers pharmaceutical companies to streamline workflows, enhance data-driven decision-making, and optimize clinical trial processes within the Trial Landscape platform. Transforming Clinical Trial Design Building upon the success of GenosAI Lite, GenosAI Pro
Read More
The New Era of Clinical Trials: Adopting Electronic Informed Consent
The clinical trials landscape continues to evolve and with it, an exponential growth in the adoption of electronic informed consent (eConsent) solutions. These solutions deliver a myriad of benefits for trial sponsors, sites and patients. However, there is still some reluctance to adopt these solutions due to perceived costs associated with implementation and training. The Importance of eConsent Clinical trials are evolving with novel designs, hybrid and decentralized
Read More
Labcorp & Hawthorne Effect Partner to Supercharge Decentralized Clinical Trials
What You Should Know: - Labcorp, a global leader in innovative laboratory services, and Hawthorne Effect, Inc., a pioneer in technology-driven clinical trial solutions, have announced a groundbreaking partnership to revolutionize clinical trials with a focus on decentralization. - This collaboration aims to unlock unprecedented levels of patient diversity, reduce site burden, and accelerate study timelines for pharmaceutical, biotech, and medical device sponsors. Decentralization: The
Read More
Clinical Trial Payments: Optimizing the Path Forward for Site Payments
Escalating competition for clinical trial sites, in tandem with other industry-wide challenges such as changing regulations; a lack of bandwidth and resources; identifying ideal sites and recruiting and retaining patients, are posing significant obstacles for sponsors and site teams within a global study portfolio, particularly in relation to clinical trial payments. Factors such as decentralized clinical trials, inflation and site attrition contribute to inconsistencies and financial confusion,
Read More